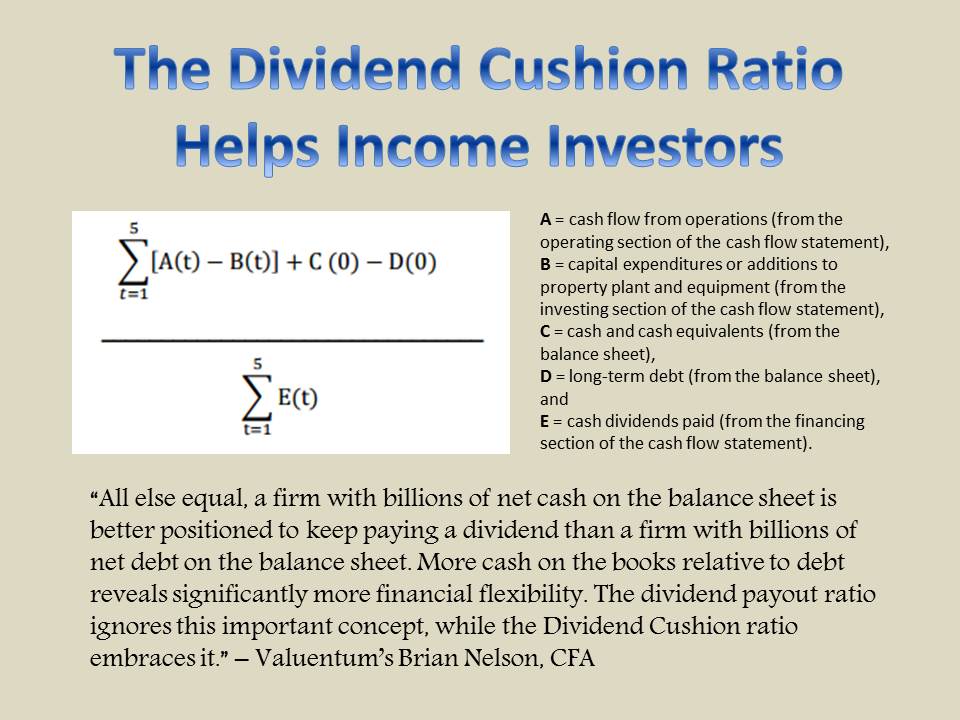
Member LoginDividend CushionValue Trap |
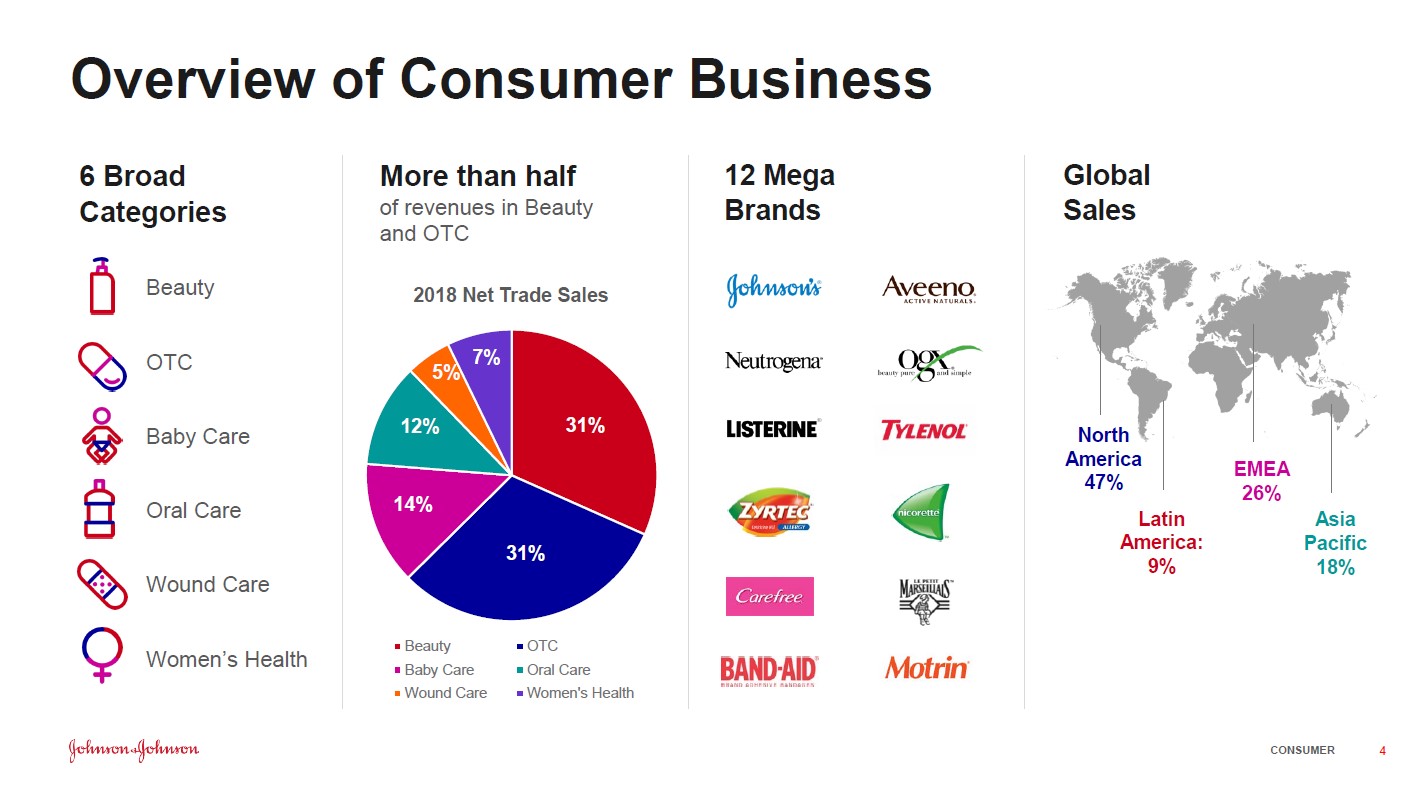
Johnson & Johnson Announces New Test Results That Reveal No Traces of Asbestos in Johnson Baby Powder Products
To gain access to the members only content, click here to subscribe. You will be given immediate access to premium content on the site.
|